INTRODUCTION
Although thrombotic events (TEs) are the leading cause of paroxysmal nocturnal hemoglobinuria (PNH)-related mortality, the risk factors predictive of TEs are not well established. Several small or previous studies reported that the proportion of PNH cells, elevated lactate dehydrogenase (LDH), age, thrombosis at diagnosis, and treatment may impact TE risk.1-5 The International PNH Registry (NCT01374360) is an observational cohort study containing the largest database of safety, quality-of-life, and outcome data from patients with PNH. Here, we analyzed patient data from the Registry to identify risk factors for TEs.
METHODS
Data from Registry patients who were untreated at enrollment, had an incident TE after enrollment, non-zero follow-up time, and with documented birthdate, sex, enrollment date, treatment status, and country, were included in this analysis. The first TEs experienced by eligible patients after enrollment were identified as TE cases; the date of the index TE event was defined as the Index Date. Up to five controls were selected from the risk set for each TE case matched on age (±5 years at Index Date), gender, country, and history of bone marrow disease (BMD). Cases that could not be matched with at least one control were excluded from the study. For covariates included in the analysis, conflicting or absent values were marked as "missing." Univariate conditional logistic regression was used to estimate odds ratios (ORs) with 95% Wald confidence intervals (CIs) of TE associated with candidate risk factors: glycophosphatidylinositol (GPI)-deficient granulocytes, GPI-deficient erythrocytes, LDH ratio, recent high-disease activity (HDA; defined as within six months prior to the Index Date, LDH ratio ≥1.5xULN, and hemoglobin <10 g/dL or at least one of the following symptoms: abdominal pain, dyspnea, dysphagia, fatigue, hemoglobinuria, and/or male erectile dysfunction), LDH ratio and number of HDA symptoms, history of TE, history of major adverse vascular event (MAVE), and recent prophylactic anticoagulant (PA) use.
RESULTS
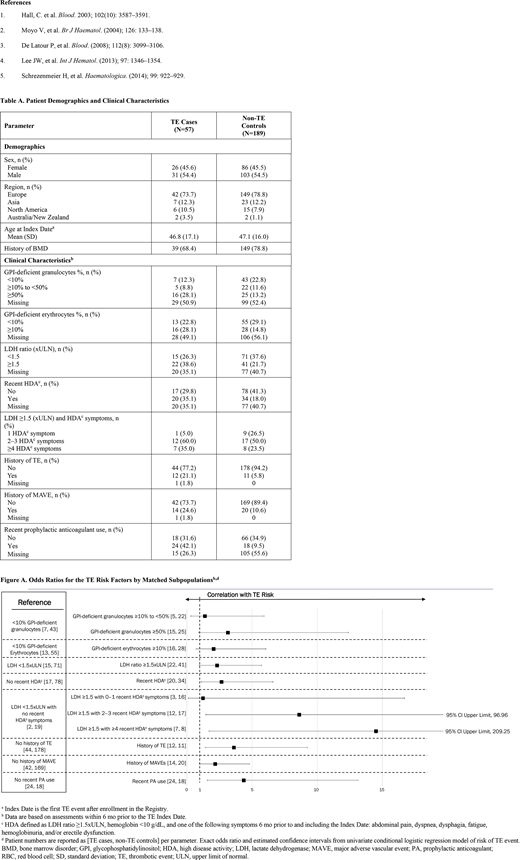
Due to the strict eligibility criteria, 57 TE cases and 189 non-TE controls met the conditions and were matched for the case-control study. The mean age at Index Date was 46.8 years for TE cases and 47.1 years for non-TE control (Table A). Cases were more likely to have a clone size of ≥ 50% GPI-deficient granulocytes, an LDH ratio ≥ 1.5xULN, recent HDA, and a history of TE or MAVE, compared with controls. From univariate analyses, the following factors were found to be associated with statistically significantly increased risk of TE: recent HDA (OR, 2.65; 95% CI, 1.10-6.61), LDH ≥1.5xULN and 2-3 HDA symptoms (OR, 8.61; 95% CI, 1.46-96.96), LDH ≥1.5xULN and ≥4 HDA symptoms (OR, 14.50; 95% CI, 1.70-209.25), and a history of TE (OR, 3.60; 95% CI, 1.41-9.24) or MAVE (OR, 2.17; 95% CI, 0.96-4.80), and recent PA use (OR, 4.35; 95% CI, 1.57-13.13) (Figure A). The strengths of this analysis include a robust study design for evaluation of a rare outcome and multiple risk factors, increased generalizability with an international patient population, and the ability to evaluate both medical history and recent clinical characteristics relevant to PNH and TE; however, not all patients had available data for each parameter assessed and the number of TE cases identified were relatively small. Despite these limitations, factors that were statistically significantly associated with increased TE risk were identified from the analysis.
CONCLUSIONS
Based on this observational PNH Registry analysis, we identified several clinical features of PNH that were associated with an elevated risk of TE, including ≥50% GPI-deficient granulocyte clone size, LDH ratios ≥1.5xULN, recent HDA, LDH ≥1.5xULN plus HDA symptoms, a history of TE or MAVE, and recent PA use compared with non-TE controls; for recent PA use, these patients were most likely at increased risk of TE, which may explain why they received treatment. Our data add to the findings of previously published studies1,4 by expanding the results to a broader patient population. These results highlight the importance and urgency of identifying and monitoring risk factors for TE in patients with PNH to inform treatment decisions.
Hoechsmann:Roche: Consultancy, Honoraria; Apellis: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Alexion: Consultancy, Honoraria, Research Funding. Peffault De Latour:Apellis: Membership on an entity's Board of Directors or advisory committees; Amgen: Research Funding; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Alexion Pharmaceuticals Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Hill:Alexion Pharmaceuticals, Inc.: Current Employment. Röth:Roche: Consultancy, Honoraria, Research Funding; Sanofi: Consultancy, Honoraria; Apellis: Consultancy, Honoraria; Alexion Pharmaceuticals Inc.: Consultancy, Honoraria, Research Funding; Biocryst: Consultancy, Honoraria; Novartis: Consultancy, Honoraria. Devos:Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees. Patriquin:Octapharma: Honoraria, Membership on an entity's Board of Directors or advisory committees; Apellis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Alexion Pharmaceuticals, Inc.: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Jain:Alexion Pharmaceuticals, Inc.: Current Employment, Current equity holder in publicly-traded company. Zu:Alexion Pharmaceuticals, Inc.: Ended employment in the past 24 months; Merck & Co.: Current Employment. Lee:Alexion Pharmaceuticals Inc.: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal